Colloid goitre is defined as thyroid enlargement without accompanying disturbance in thyroid function. This is a common pathology, frequently found in clinical practice during a physical or ultrasound examination. Colloid goitre has been classified as nontoxic goitre according to the updated International Classification of Diseases (Table 1).1 Colloid goitre is also known as endemic goitre, simple goitre, nontoxic uninodular goitre, nontoxic multinodular goitre or nodular hyperplasia. They are benign lesions; however, on the ultrasound image, they can mimic malignant lesions.2,3

We conducted searches on the PubMed database for articles published between September 1967 and August 2020 to provide a summary of key issues and updates related to colloid goitre. Search terms included: ‘colloid goitre’, ‘diffuse hyperplasia’, ‘nodular thyroid’, ‘nontoxic goitre’, ‘endemic goitre’, ‘simple goitre’, ‘nontoxic uninodular goitre’, ‘nontoxic multinodular goitre’, ‘nodular hyperplasia’, and ‘colloid nodule’. In addition, the search was expanded to include terms such as ‘thyroid nodule’, ‘thyroid pathology’, ‘treatment of thyroid pathology’, ‘thyroid ultrasound’, ‘thyroid malignancy’, ‘thyroid cytopathology’, ‘thyroid evaluation’ and ‘thyroid nodule management’. Unrelated articles were excluded. This article provides an overview of the aetiology, epidemiology, pathophysiology, histopathology, clinical manifestations and ultrasound features of colloid goitres, as well as differential diagnosis and management.
Aetiology and epidemiology
There are many mechanisms and aetiologies that cause colloid goitre (Table 2).2,4 A number of possible factors leading to colloidal goitre include foods that block the hormonal synthesis, mutations in thyroid-stimulating hormone (TSH) receptors, globulin stimulation of thyroid development, growth hormone, insulin-like growth factor 1 (IGF-1) and genetic factors.5,6 An iodine-deficient diet is also known to lead to colloid nodular goitres. There is evidence that iodine supplementation can decrease the incidence of goitre in these people; however, some cases of longstanding endemic goitres do not always regress with iodine supplementation.4 It has also been suggested that thyroid function control factors, such as C cells, can alter iodine levels, leading to goitre.7 According to some studies, iodine deficiency is associated with a 5–10% prevalence of goitre.8,9 The peak age for the onset of goitre is 35–50 years, and the ratio of women to men affected by goitre is 3:1.9 There is no correlation between race and the prevalence of goitre.8-10

Pathophysiology and histopathology
The most important factor of colloid goitre appearance is a reduction in TSH stimulation for a prolonged period of time.11 Pathologically, these are often known as hyperplastic and colloid nodules, sometimes they may also be described as adenomatous. The majority of cystic thyroid lesions are hyperplastic nodules that have undergone extensive liquefactive degeneration by the time they are detected. In cases of diffuse goitre, there will be follicular cell hyperplasia.12 In chronic settings with continued TSH stimulation, some follicles become autonomous and secrete hormones that will depress other areas, which then involute. This leads to multinodular goitre with areas of focal hyperplasia and areas of involution and fibrosis.2,11,13
The thyroid gland stores the thyroid hormone in an acellular glycoprotein called colloid. Colloid is present in a variety of forms, from watery and pale to thick and dense, resulting in a broad spectrum of manifestations in cytological preparations. Usually, the colloid is more watery and paler the more active the gland is. The less active it is, the denser the colloid. It is more likely that an abundant colloid is associated with benign nodules.14
Histologically, the initial stage of colloid goitre is cellular hyperplasia of the thyroid acini, followed by a micronodular and macronodular formation, which is often indistinguishable from normal thyroid parenchyma, even on histological examination. True thyroid epithelial cysts are rare.15 Hyperplastic nodules are often subject to liquefying degeneration, accumulating blood, serous fluids and colloidal substances. In this cystic-degenerative process, calcification can occur, which is often coarse and peri-nodular.16
Patient history and physical examination
If a thyroid lesion is palpated, it can be referred to as a nodular goitre, clinically. When there are two or more unilateral or bilateral nodules appearing in the lobes of the thyroid gland, the lesion may be classified as a multinodular goitre. Most patients with colloid goitre are asymptomatic.17 The swelling may be discovered accidentally by the patient or others. On physical examination, there will be a central neck swelling that could be smooth or nodular and moves with swallowing. By palpation of the thyroid, the location, size, consistency and mobility of the nodules can be determined.18 Multinodular goitre is consistent with benign disease, especially if all nodules have a similar consistency. Some individuals may have compressive symptoms such as dysphagia, dyspnoea and hoarseness of voice due to mechanical compression of the oesophagus, airway and laryngeal nerves by the nearby huge goitre. Goitre can push the trachea or extend retrosternally.19 The vocal cords must be examined in cases of hoarseness, or previous surgical intervention. Large thyroids may compress the neck veins, leading to facial congestion and discomfort. Pain is rare, but it may be severe and incremental when there is bleeding in a nodule, and may be associated with sudden changes in the goitre. Any cervical lymphadenopathy that suggests malignancy requires more exclusion workup. In addition, colloid goitre in ectopic thyroid tissue has also been reported.17
Ultrasound
Diffuse hyperplasia
On ultrasound, a normal thyroid exhibits an isoechoic homogeneous structure with a fine granularity that does not exceed 1 mm. Heterogeneous echostructure is recorded when there is a difference in echodensity compared with the normal background. The concept of a nodular goitre does not always correspond to its morphological definition.20 In ultrasound practice, this term is described as a thyroid lesion, of any size, containing a capsule that could be determined by any modes of visualisation. About 80–85% of all thyroid abnormalities are diffuse hyperplasia of the thyroid gland.21,22 Diffuse hyperplasia embodies the following ultrasound attributes:
- increased thyroid volume;
- homogeneous isoechoic echostructure accompanied by a middle- or fine-grained pattern;
- regular margins with clear and accurate borders; the contours of the poles may be rounded; and
- Doppler imaging may show a negligible symmetric increase in the number of vessels within the thyroid gland with a uniform distribution, or a normal colour spectrum may usually be visible (Figure 1).23,24
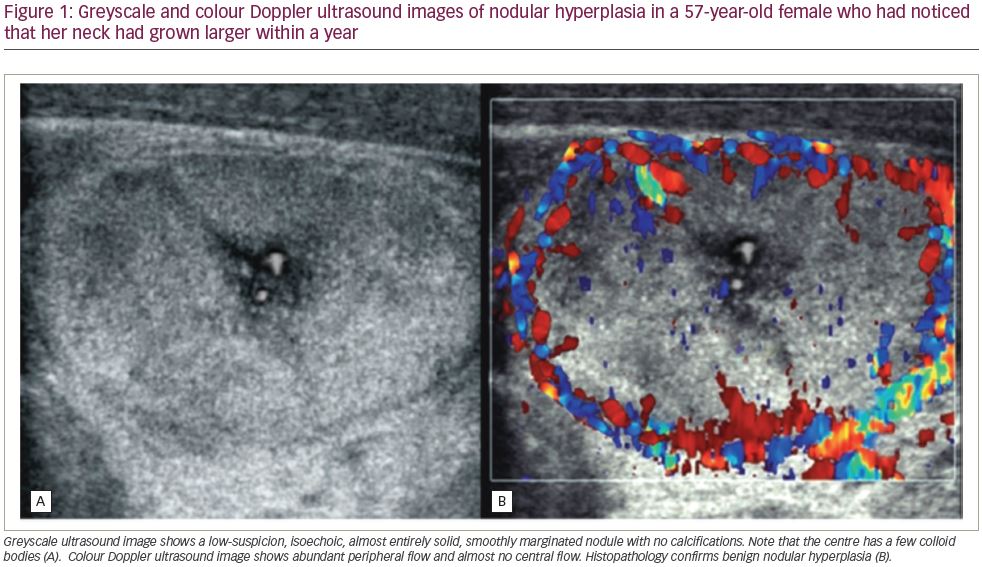
Doppler modalities, in most cases, do not significantly complement a greyscale ultrasound.21,25
The degenerative changes of the goitre nodules are equivalent to their sonographic appearance. Pure anechoic zones are caused by sera or colloid fluid. Echogenic fluid or moving fluid levels are equivalent to bleeding.26 Bright echogenic foci with comet-tail artefacts are likely to be caused by microcrystals or aggregates of colloid substances, which may also move slowly, like snowflakes, descending within the fluid pool.27 Intracystic septations are likely to correspond to attenuated strands of thyroid tissue and appear completely avascular on colour Doppler ultrasound. Degenerative processes of hyperplasia nodules may also lead to the formation of calcifications, which may be either thin, peripheral shells; or coarse, highly reflective foci with associated acoustic shadows scattered throughout the gland.24,28,29
Intracystic solid projections, or papillae, usually containing colour Doppler signals, may appear similar to rare cystic papillary thyroid carcinoma. In some cases, ultrasound imaging cannot differentiate the septations of colloid nodules from the vegetations seen in papillary carcinomas; before moving to aspiration cytology studies, contrast-enhanced ultrasound with second-generation microbubbles and nondisruptive imaging can be used. Benign septa and intracystic contents do not show enhancement, whereas malignant vegetations show intense enhancement in the arterial phase, with relatively fast washout.24,25,29
Colloid nodular goitre
To approach nodular thyroid, the clinician should assess whether the patient has thyrotoxic thyroid, subclinically toxic thyroid, hypothyroid or euthyroid, by quantifying TSH, free T4 and T3. Ultrasound must then be conducted to assess the lesions.23 Colloid nodules can be hypoechoic, isoechoic or hyperechoic compared with adjacent normal thyroid tissue. They may contain fluid or mixed components that create multiple interfaces between cells and colloids. Sometimes, a sponge-like or honeycomb pattern may also present.30 Ultrasound characteristics of common colloid nodules include well-defined margins and an intact capsule of lesions, and the thyroid gland capsule should be preserved (Figure 2).22,27,29 Doppler ultrasound can be used to determine whether there is internal vascular flow. When the nodule is isoechoic or hyperechoic, a thin peripheral hypoechoic halo is typically seen, most likely caused by perinodular blood vessels and mild oedema or compression of the adjacent normal parenchyma. Perinodular blood vessels are typically detected, or intra-nodular vascularity can also be seen, with Doppler ultrasound.20,28

A comet-tail artefact is another common finding, and is caused by reverberation echoes between the two interfaces. The reflection of sound waves out of the crystal leads to a bright spot. It is displayed as a hyperechoic item with a triangular and tapered form. In contrast to soft-tissue calcification, suspended crystals vibrate under the action of ultrasonic energy.28 The vibration forms sound waves that return to the transducer after the first recording signal creates a comet-tail image. These comet tails, also referred to as the ring-down artefact or step-ladder artefact, help distinguish between the typical benign densities that occur in a colloid nodule and highly suspicious microcalcifications. If there is a single comet tail in a small colloid cyst, this is called a ‘cat’s eye’.20,21,23
Long-lasting colloidal nodules may present distinct calcifications in the periphery of the lesion. They are often calcified in the form of a shell-shaped or an eggshell calcification. Peripheric calcification is significantly different from the large crude calcification and microcalcifications often found in thyroid cancer. The combination of colloid nodules with cysts, adenomas or thyroid cancer is rare.31 In some cases, the comet-tail artefact can also occur in the resolution of haematomas and has been rarely described in papillary carcinoma.25 This finding suggests that some malignant microcalcifications may also look like small comet-tail artefacts.32,33 Several studies indicate that comet tails are typically within, or at the edge of, the follicle; whereas microcalcifications occur scattered in solid tissue.34,35 Occasionally, it can be difficult to distinguish the density of a comet tail from a microcalcification, leading to divergent opinions on how reassuring it is to observe the density of a comet tail within a nodule. Studies of thyroid ultrasound have shown that the harmonic option (display mode on ultrasound machine) improves the structural image quality of the colloid nodules in 75–85% of cases.22,24,27,29
Tahvildari et al. studied the properties of punctured echogenic reflectors in papillary carcinomas and explained that microcalcifications that arise from a variety of causes, including psammomatous calcifications, dystrophic calcifications and eosinophilic colloids.35 Therefore, the terms ‘punctate echogenic foci’ or ‘micro-reflectors’ may be more specific terms. Nodules with large comet-tail artefacts, especially those around a cystic nodule, are almost always a benign lesion.34 A biopsy can be remarkably avoided in most of these lesions unless other suspect features are present.27 Therefore, radiologists need to systematically master the characteristics of the thyroid nodules to produce a reliable result. Features that make nodules suspicious are: taller-than-wide in shape, solid or almost completely solid composition, have extra-thyroidal extensions, are very hypoechoic, have microcalcification and have hypervascularity (Figure 3).31,35 In these situations, fine-needle aspiration cytology under ultrasound guidance is recommended.28,36

Treatment and prognosis
Colloid goitre, as well as nontoxic goitre, usually progresses slowly and is nonsymptomatic; therefore, it only requires follow-up, and not treatment.37 Medical treatment for nontoxic goitre is a matter of debate, as it produces little or no results in long-term goitres. For patients with compression symptoms or complications, non-emergency surgical treatment is indicated,38,39 which must include total, or near-total, gland excision. Surgical treatment has the advantage that the recurrence rate is relatively low.40–42 Radioiodine ablation is another modality of therapy.43,44 Complications including hypoparathyroidism and recurrent laryngeal nerve injury can occur during or after thyroidectomy.45 Additionally, patients who undergo total thyroidectomy require life-long thyroid supplements. There are many algorithms for the diagnosis and treatment of thyroid disease in general, and colloid goitres in particular.1,43,46,47
Differential diagnosis
Colloid goitre diffusion, or nodular goitre, must be differentiated from other causes of a goitre. The most important issue here is the elimination of malignancy.48 Thyroid malignancies that must be excluded include thyroid lymphoma, papillary thyroid cancer, follicular thyroid cancer and medullary thyroid cancer.49–51 Anaplastic thyroid cancer is usually manifested as a large and fixed swelling. Another cause that must be differentiated from nontoxic goitre is inflammatory goitre.52,53 Hashimoto thyroiditis, Riedle thyroiditis and De Quervain thyroiditis are important inflammatory goitres that need be differentiated from colloid goitre.54,55
Conclusions
Colloid goitres are a common, benign lesion of the thyroid that includes diffusion or a nodular pattern. They must be differentiated from other causes of goitre, especially malignancy. Accurate diagnosis requires a general knowledge of the condition. Patient history and physical examination, focusing on features suggestive of malignancy, are very important in the initial evaluation. Thyroid ultrasound and serum thyrotropin are the main methods for assessing colloid goitre and excluding other thyroid lesions.













