Tuberculosis (TB) is a major public health concern in low- and middle-income countries (LMICs) of Asia and Africa, which have a high burden of human immunodeficiency virus (HIV) infection and malnutrition. It is epidemic in these regions being associated with high rates of morbidity and mortality.1 Though, TB incidence is declining at about 2% per year globally these regions still have many high TB burden countries.2
TB typically affects the lungs in adults, with a predilection to the upper lobes and varied manifestations. Extrapulmonary TB is usually secondary to pulmonary TB. Children are more likely to have primary complex TB, TB lymphadenitis, central nervous system TB, and when compounded with malnutrition, the miliary form of TB. Though uncommon, involvement of endocrine glands may be a part of disseminated infection or miliary TB or seen in patients with previous history of TB, involving either the lung, bones, joints, intestines, or genitourinary tract. In recent times the incidence of endocrine involvement has been low due to effective anti-TB therapy.
In most patients, overt endocrine manifestations are unusual and infrequent. One must be vigilant for these endocrinopathies in the presence of risk factors, namely previous TB, HIV infection, history of immunosuppressive treatment, hemodialysis, gastrectomy, jejunoileal bypass, or diabetes. In many instances, endocrine abnormalities do not always reflect direct infection of the gland, but may be the result of a physiological response or a consequence of therapy for TB. The most commonly involved endocrine glands in TB are the adrenals followed by pituitary, and hypopituitarism due to intracranial tuberculoma or tuberculous meningitis has been reported.3 Thyroid and other endocrine glands are less commonly affected. TB also imparts a cytokine-mediated immuno-inflammatory state with altered immune–endocrine response, which has an impact on clinical and metabolic status as well as innate and adaptive immune response to Mycobacterium tuberculosis.4
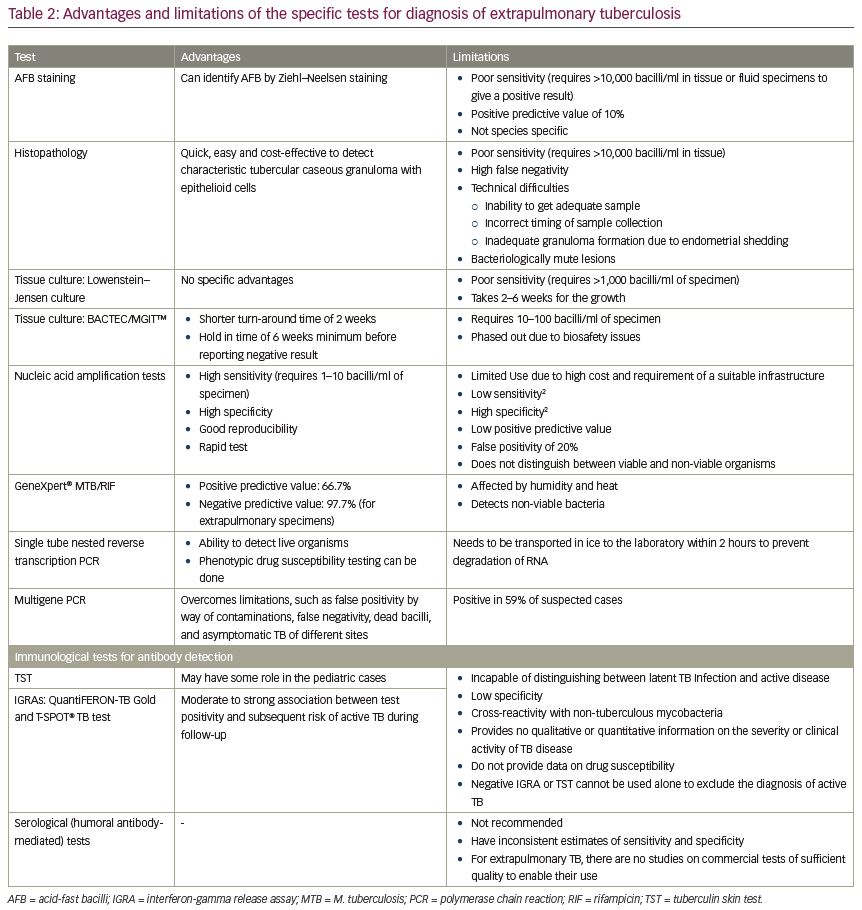
Absence of overt symptoms and signs is a challenge to diagnosis; however, if suspected, magnetic resonance imaging (MRI), computerized tomography (CT), and CT-guided biopsy, with histopathology and microbiological testing should be done (Table 1). Specific tests include acid-fast bacilli detection, culture sensitivity and nucleic acid amplification tests (Table 1).5 The advantages and limitations of the diagnostic tests are elaborated in Table 2.6

The mainstay of modern therapy for TB is anti-TB treatment. Table 3 highlights the first-, second-, and third-line drugs with their dosage regimes.7 A combination of first-line antibiotics is used for treatment and is usually given for a period of 6–9 months. The treatment regimen consists of an initial 2-month treatment phase of four drugs followed by a continuation phase of two drugs for either 4 or 7 months (Table 4).7,8 Second- and third-line drugs may be required for multidrug-resistant TB or extensively drug-resistant TB.2 Once a TB treatment regimen is initiated, it is important to assess and ensure adherence to therapy. Inadequate treatment can lead to failure to respond, persistent infectivity, development of drug resistance, and disease recurrence. It is important to monitor the patient for adverse reactions and response to treatment to determine efficacy and drug resistance. Endocrine and metabolic abnormalities do not always manifest with symptoms and signs but may require anti-tuberculosis therapy or specific interventions. The aim of the review is to identify both the direct and indirect impact of M. tuberculosis infection on endocrine glands and metabolic function so that when suspected we could diagnose and treat these.


Search methods
An electronic literature search was performed in PubMed Medline (1966–2020) using search terms “endocrine and metabolic aspects of tuberculosis”, “adrenal insufficiency”, “thyroid dysfunction”, “pituitary dysfunction”, “disorders of calcium and vitamin d metabolism”, “diabetes mellitus”. A total of 221 articles was retrieved. Appropriate cross references were then manually searched.
Tuberculosis of the adrenal gland
TB accounts for about 7–20% of cases of Addison’s disease.9 Five percent of the patients with disseminated TB develop clinically significant adrenal insufficiency and the incidence is higher in LMICs.10 Adrenal involvement is reported in 6% of cases in an autopsy series.11 TB infection of the adrenal gland is a result of hematogenous spread of the infection, most likely from pulmonary or genitourinary tract infection.12 No active extra-adrenal TB is seen in about 12% of patients with adrenal TB.13
Adrenal TB is often asymptomatic, and clinical features due to deficiency of production of glucocorticoids, mineralocorticoids, and androgens, appear only at a later stage, when more than 90% of the gland has been destroyed.14 Patients usually present with vomiting, diarrhea, generalized weakness, hyper-pigmentation, malaise, anorexia, weight loss, and postural hypotension. A history of either active TB elsewhere or a positive tuberculin skin test, may suggest a possibility of adrenal gland TB. Adrenal insufficiency in TB, in most cases, is due to direct involvement of the adrenal gland, which may initially be enlarged with extensive epithelioid granulomas and caseation, but as the disease progresses, with fibrosis it may become normal or smaller in size.15 Calcification is evident in about 50% of cases.16 At times, the adrenal gland can be enlarged in pulmonary TB without adrenal involvement, possibly related to stress cortisol response and inflammation.14
Diagnosis
Recent improvements in imaging techniques and endocrinologic assays for adrenal function have provided greater insight into adrenal involvement in TB. Investigations usually reveal hyponatremia with hyperkalemia, low serum cortisol, and elevated serum adrenocorticotropic hormone levels diagnostic of Addison’s disease. An 8 am fasting cortisol level of <3 mcg/dl (83 nmol/l) suggests adrenal insufficiency. Cosyntropin or adrenocorticotropic hormone stimulation test is the most specific test for diagnosing adrenal insufficiency.17
On imaging, unilateral or bilateral non-calcified adrenal enlargement is seen on CT scan with active infection. Areas of lucency reflecting caseous necrosis with peripheral rim enhancement may be demonstrated with contrast-enhancement.18–21 Atrophy and calcification is usually seen in chronic infection, with shrunken adrenal glands, calcific areas, and irregular margins. However, CT scans often do not provide a definitive diagnosis.18 A cold abscess may result in a mass effect on imaging, with similar findings noted on MRI scans; however, calcifications in chronic cases are better seen on CT scans. MRI findings include hypointense or isointense areas on T1- and hyperintense areas on T2-weighted images. There are limited data available on positron emission tomography scans; however, one study has reported fluorodeoxyglucose enhancement similar to malignant lesions.22
A microbiological sample and tissue biopsy may be needed to rule out other causes of adrenal enlargement, such as malignancy, hemorrhage, fungal infection, amyloidosis, sarcoidosis, adenoma, hemangioma, and hyperplasia. CT-guided needle biopsy will help in confirming the diagnosis by the presence of caseating or noncaseating granulomas with glandular enlargement, adrenal destruction due to inflammation, or adrenal atrophy and fibrosis in chronic infection.11 Epithelioid granulomas are less frequent than in extra-adrenal foci, reflecting local production of anti-inflammatory steroids.23 Though calcification of adrenal gland is common, it is not a specific finding. Molecular diagnosis with culture-sensitivity of aspirated material for M. tuberculosis is diagnostic; however, specimens may not always be positive (Table 2).
Management
The drugs used and the standard anti-TB treatment regimen are as highlighted in Tables 4 and 5, and these are generally safe and well tolerated. For acute adrenal insufficiency, initial management involves administration of intravenous hydrocortisone (50–100 mg) every 6 hours, followed by maintenance doses of oral steroids at 15–20 mg per day.24 Most common oral steroids used are hydrocortisone or prednisone.24 Oral steroids are given three times daily, with the distribution of dose as follows: 10 mg in the morning, 5 mg at lunch time, and 5 mg in the early evening—corresponding to the circadian rhythm.25–27 Patients on anti-TB therapy may need a higher dose of oral steroids, as rifampicin induces the cytochrome P450 enzyme system, causing increased metabolism of glucocorticoids.28 Adrenal crisis may result if steroid replacement therapy is inadequate.29 Mineralocorticoid therapy is also essential in Addison’s disease. Adrenal size and function improve if anti-TB therapy is started early in the course of the disease, before its destruction.30 In patients where adrenal gland destruction is substantial, treatment will reduce adrenal gland size, but may not result in full recovery of adrenal function.29
Tuberculosis of the thyroid gland
The thyroid gland is rarely involved directly by M. tuberculosis due to the intrinsic properties of the thyroid gland, which include the presence of colloid, high blood flow with excess bactericidal iodine, increased phagocytosis associated with hyperthyroidism, and extensive lymphatic and vascular supply.31
With current anti-TB regimens, the prevalence rate of thyroid involvement is 0.1–1%, where the diagnosis was established after fine-needle aspiration and cytology (FNAC) of thyroid tissue sampled for any indication.32–34 Two publications, show thyroid involvement in 0.43% of FNAC samples in India and 0.6% from thyroidectomy specimens in Turkey.35,36 The exact prevalence of the infection is not known, and varies between 0.1 and 1.15%.37 The incidence of thyroid TB is 0.2% in chronic thyroiditis and 7–14% among those with miliary tuberculosis.38–40
The spread is usually via the hematogenous route, and very rarely can be directly from adjacent active laryngeal or lymph node foci. Primary involvement of the thyroid gland with TB has also been reported.41
Diagnosing thyroid gland TB may be difficult as thyroid function tests are rarely deranged and no specific symptoms are present. A few cases in initial stages can present with hyperthyroidism due to parenchymal damage and increased release of thyroid hormones. Significant destruction of the gland may later cause hypothyroidism.42–45 Most patients usually present with a painless thyroid nodule or diffuse goiter and lymphadenopathy with constitutional symptoms such as weight loss and fever of undetermined origin. Very rarely, a thyroid mass, thyroiditis, or an acute or cold abscess with or without a discharging sinus may be present. Caseation may or may not be present. Rarely, chronic fibrosing TB of the thyroid is seen with miliary disease.39 Histopathology shows presence of epithelioid granulomas, with central caseation, Langhans giant cells, and peripheral lymphocyte cuffing.42–44
One needs to differentiate tuberculous thyroiditis from bacterial thyroiditis. Although in patients with tuberculous thyroiditis the duration of symptoms is longer, they are less likely to have pain, thyroid tenderness, and fever, seen in bacterial infection. These patients can also develop dysphagia, dysphonia, or recurrent laryngeal nerve palsy due to compression of adjacent structures or fibrosis. Tuberculous thyroiditis is a diagnosis of exclusion for solitary cold thyroid nodules with normal thyroid function, especially in patients who are tuberculin-positive. On ultrasonography, tuberculous thyroiditis has a wide range of appearances from solid and heterogenous masses to cystic or hypoechoic lesions.30 This varied presentation makes it challenging to diagnose. CT and MRI findings are non-specific but may show evidence of intermediate signals on T1- and T2-weighted imaging or peripheral rim enhancement consistent with abscess.46,47
Cytology and microbiology using FNAC is preferred for excluding carcinoma or other granulomatous lesions like sarcoidosis, syphilis, and Hashimoto’s thyroiditis.32–34,36 In countries of high prevalence in South East Asia and Africa, with a suspected diagnosis, treatment may be started in the presence of epithelioid granulomas with caseation, even in the absence of bacterial confirmation.32 Management includes a combination of surgery and anti-TB therapy. When a definitive diagnosis of thyroid involvement due to M. tuberculosis is made, sole treatment with standard anti-TB therapy, has resulted in favorable recovery of both the lesions and the abnormal thyroid function, if present.45 Treatment regimens are identical to those outlined in Table 4 for a duration of 6 months. Recurrence and failure rate with anti-TB therapy are about 1%, due to drug resistance. Thyroxine supplementation is indicated in the presence of hypothyroidism.48 Surgery has a limited role in cases of unclear diagnosis or with suspicion of malignancy. In the presence of a tuberculous cold abscess, surgical drainage is required to avoid further destruction of thyroid tissue and consequent hypothyroidism.
Thyroid function abnormalities without gland involvement may rarely be seen in patients with active TB elsewhere. Following treatment initiation, there could be an elevation of serum free triiodothyronine and total triiodothyronine;49 with serum T4 and thyrotropin usually unaffected.50
Goiter and hypothyroidism can be seen with the use of ethionamide and/or para-aminosalicylic acid as a second-line therapy.51–54 Induction of hepatic microsomal enzymes by rifampicin may enhance the extrathyroidal metabolism of thyroid hormones.55,56 These effects of anti-TB therapy, especially when rifampicin, ethionamide, and/or para-aminosalicylic acid are used, makes it prudent to monitor thyroid function along with liver function.
Euthyroid sick syndrome is the presence of abnormal thyroid function tests in patients with non-thyroidal illness, like TB, without pre-existing hypothalamic-pituitary and thyroid gland dysfunction. The incidence of euthyroid sick syndrome in TB ranges from 63–92% and serves as an indicator of severity of illness.57 Cytokines such as tumor necrosis factor-alpha play a role in development of this syndrome.58 The abnormal thyroid function tests are completely reversible after recovery from TB.
Tuberculosis of the pituitary gland
Involvement of the pituitary gland is rare and its incidence has reduced from 4% of all intracranial lesions in the pre-antibiotic era59 to 0.15% with modern-day anti-TB therapy.60,61 The route of spread is usually hematogenous, though it may also be due to local extension from adjacent anatomical sites, namely the meninges, sphenoid sinus, cavernous sinus, and the skull.62 These inflammatory granulomatous lesions of the pituitary gland can behave as tumors being denoted as non-functioning sellar masses. The lesions can range in size from 2–12 mm. They can also undergo caseation forming a pituitary abscess, or hemorrhagic infarction.63 Adjacent areas, such as the hypothalamus and pituitary stalk, also can be involved. It is very difficult to differentiate from the symptoms, whether the hypothalamic–pituitary dysfunction is due to direct pituitary or hypothalamic involvement or the involvement of both pituitary and hypothalamus. The clinical presentation varies; patients can be asymptomatic with subtle endocrine abnormalities or may present with fever, headache, and visual complaints, with or without selective hypopituitarism (seen in 20% of cases).64–66
Anterior pituitary dysfunction can occur later with or without central diabetes insipidus due to the compressive effect of the granulomatous lesion.67 The presentation may vary with age and severity of the disease: there could be growth retardation, hypogonadism, galactorrhea, amenorrhea, diabetes insipidus, and at times, panhypopituitarism.62,65–69 In males, hypopituitarism accompanied by hyperprolactinemia can result in decreased libido.63 In females, significant reduction in female sex hormones (progesterone and E2) results in disordered menstruation or amenorrhea.70 The most common abnormalities in pituitary function found in patients with tuberculous meningitis are hyperprolactinemia (49%), cortisol insufficiency (43%), hypothyroidism (31%),71 and hypogonadotropic hypogonadism with low testosterone and gonadotropin levels (29%).71–73 Of the different abnormalities, growth hormone deficiency is the commonest sequalae.74 Timely diagnosis and administration of anti-TB therapy can reverse the endocrine dysfunction.
Hypothalamic–pituitary dysfunction may also occur in association with tuberculous meningitis which represents 1% of all cases of TB.75 The pathological process in tuberculous meningitis results in formation of dense inflammatory exudates at the base of the brain involving the ambient cistern and the pontine cistern.76 Obliterative vasculitis with damage to hypothalamic–pituitary axis is also described. Both pathologies can result in hypothalamic–pituitary dysfunction with hormonal abnormalities, which improve in most patients with treatment of tuberculous meningitis.76 Tuberculous meningitis-related hypopituitarism is one of the most under-recognized and under-reported conditions.72
Pediatric tuberculous meningitis results in hypothalamic–pituitary dysfunction in 20% of patients, and can manifest even several years after recovery.77 The late endocrine sequalae in children include obesity, precocious puberty, diabetes insipidus, Frohlich syndrome, and growth retardation.78 Often, asymptomatic secondary hypoadrenalism due to pituitary dysfunction may remain undiagnosed in children, but may cause a fatal adrenal crisis during physical or psychological stress later in life. However overall, the incidence of hypothalamic–pituitary dysfunction in children is less frequent as compared to adults.77
Hypothalamic–pituitary dysfunction may also result from the weight loss and chronic illness that affect the hypothalamic gonadotropin-releasing hormone pulse generator.79 Disruption of hypothalamic–pituitary–gonadal axis can occur due to low leptin levels seen with weight loss and cachexia as a result of TB.80 With weight gain after anti-TB therapy, leptin levels rise and activate the hypothalamic–pituitary–gonadal axis with the return of normal function.81
Pituitary gland TB should be suspected in patients who have signs or symptoms of hypopituitarism with a history of TB elsewhere in the body. Assessment of endocrine function and radiological evaluation using MRI and CT scan will assist diagnosis, with the latter demonstrating calcification in the region of the sella turcica82 or the presence of intrasellar tumor. Thickening and nodularity of pituitary stalk, with or without extension into the sphenoid sinus, may be noted on CT or MRI.69,83,84 A TB pituitary abscess on MRI appears isointense to hypointense on T1-weighted images and hyperintense on T2-weighted images. But one must remember that, these signal characteristics are non-specific and can overlap those of pituitary adenomas and other inflammatory and granulomatous lesions.63 Tuberculous meningitis is usually diagnosed by lumbar puncture.
It is often difficult to histopathologically demonstrate caseating granulomas or acid-fast bacilli in pituitary disease or abscess. In most cases, the diagnosis is based on clinical features of hypopituitarism with MRI and biopsy, and if possible, confirmation with cultures should be done. The differential diagnoses of pituitary gland TB includes lymphocytic adenohypophysitis, sarcoidosis, Wegener’s granulomatosis, idiopathic giant cell granulomatous hypophysitis, Takayasu’s disease, Langerhans cell histiocytosis, syphilis, giant cell granuloma, Cogan’s syndrome, and Crohn’s disease.64
Treatment includes 2 months of rifampicin, isoniazid, pyrazinamide, and ethambutol followed by up to 10 months of rifampicin and isoniazid.85 It is important to consider a lower efficacy of drugs due to the presence of the blood–brain barrier. Hormone replacement therapy may be necessary in some cases.86 Most lesions tend to resolve after standard anti-TB therapy (Table 5).87 Surgery is rarely indicated as a treatment modality, except for obtaining biopsies to confirm diagnosis in ambiguous cases. Trans-sphenoidal surgery may be necessary when there is radiological evidence of mass extension with established hypopituitarism. Surgery should be performed carefully to avoid excessive resection and to prevent hormonal deficiencies following surgery. Surgical findings include presence of a thickened hypophyseal stalk, a greyish firm mass with caseation, and a thickened dura.88,89


Involvement of other endocrine glands
Tuberculosis of the thymus
Thymic involvement due to TB, though infrequent, can occur from direct infection. However, the thymic microenvironment also may be altered due to cytokine and immuno-hormonal alterations during active TB infection. This may contribute to a deficient control of infection and disease immunopathology.90 These patients present with non-specific general symptoms like fever, lethargy, loss of appetite, weight loss, retrosternal chest pain, cough, and progressive dyspnoea.91
Tuberculosis of the parathyroid gland
The parathyroid glands are not usually directly infected with M. tuberculosis. Pulmonary TB can be associated secondary hyperparathyroidism, which causes activation of osteoclasts, leading to increased bone resorption and consequently, hypercalcemia. This may be associated with or without excess production of 1,25-dihydroxyvitamin D3.92–94 In patients with concurrent vitamin D deficiency, hypercalcemia may not be present but may be unmasked after vitamin D supplementation.95 TB has been reported to be associated with persistent hypercalciuria, a low calcidiol level, and higher calcitriol level, which reverted to normal after anti-TB therapy (Table 5).96,97
Anti-TB therapy, particularly rifampicin and isoniazid, can decrease vitamin D levels by accelerated metabolism, and increase parathyroid hormone. Low vitamin D levels can mask hyperparathyroidism and hypercalcemia until anti-TB therapy is completed.98 Vitamin D supplementation may be needed.
Tuberculosis of the pancreas
TB of pancreas is very rare, even in regions of high TB prevalence, and occurs more commonly in males.99 Most cases of pancreatic TB are secondary to TB infection elsewhere in the body and the incidence may be higher with disseminated and miliary TB. Though most patients are asymptomatic, some may manifest with an abdominal mass and a cold abscess.100 Few may even present with obstructive jaundice, pain, fever, weight loss, or gastrointestinal bleed. Very limited data are available on pancreatic TB and its effects on endocrine and exocrine function of the pancreas.100
Biopsy or FNAC usually confirms the diagnosis of TB, with differential diagnoses being abscess or malignancy of pancreas.101–104 Ultrasonography, CT scan, and endosonography-guided biopsy are the recommended diagnostic techniques, as direct histopathological examination is the best way of diagnosing TB. The diagnosis of pancreatic TB should always be considered in young patients who present with non-specific symptoms and a pancreatic mass with peripancreatic lymphadenopathy on imaging studies. Most patients achieve complete cure with standard anti-TB therapy (Table 5).105
Tuberculosis of the pineal gland
TB of the pineal gland is rare and is more often seen in children. In patients with any TB, mean melatonin and 6-hydroxymelatonin sulphate concentrations are significantly lower than the controls subjects.106 Patients with pulmonary TB exhibit impairment of the circadian rhythm, which is more distinct in disseminated TB.107 Circadian rhythm is either misaligned or phase shifted, or is associated with reduced mean levels and amplitude of fluctuation of melatonin secretion.107
Gonadal involvement
In females, gonadal dysfunction is seen in about 56% of patients with genital TB.108 In males, isolated TB orchitis is very rare and is usually the result of direct extension from the epididymis.109 Anti-TB therapy can affect the metabolism of sex hormones with a moderate increase in estradiol and estrone levels. Though testosterone levels are unaltered, an increase in androstenedione levels has been noted. Among anti-TB drugs, rifampicin was responsible for the alteration in steroid hormone metabolism.110 Such interactions of anti-TB therapy with steroidal hormones in the female can result in contraception failure due to increased steroid degradation.
Metabolic derangements in tuberculosis
Syndrome of inappropriate antidiuretic hormone secretion with hyponatremia
Hyponatremia is one of the most common electrolyte abnormalities observed due to inappropriate ectopic secretion of antidiuretic hormone in patients with pulmonary, miliary, and central nervous system-related TB.111,112 The exact mechanism of syndrome of inappropriate antidiuretic hormone secretion (SIADH) associated with pulmonary TB is poorly understood.112 Tubercular meningitis and the mass effect of tuberculoma on the pituitary stalk or pituitary gland itself and overt or subclinical adrenal insufficiency can also cause hyponatremia in patients with TB.74,113 In patients with HIV infection with TB, the incidence of hyponatremia is higher.114 Anti-TB drugs like ethionamide also cause SIADH and this may not be dose related.115 SIADH should be considered in the presence of hyponatremia with low serum osmolality, a normal acid-base state, urine osmolality >100 mOsm/kg, and urine sodium concentration >40 mEq/l.
Tuberculosis and diabetes mellitus
Patients with diabetes have an increased tendency to develop TB due to impaired cell-mediated immunity, renal failure, micronutrient deficiency, and pulmonary microangiopathy.116,117 TB, which is a chronic infection, has a persistent inflammatory state, with an immune response associated with reactionary hyperglycemia, due to increased production of stress hormones like epinephrine, glucagon, cortisol, and growth hormone, which act synergistically.118 The occurrence and severity of stress hyperglycemia is related to duration of illness, severity of disease, degree of mycobacterial burden, and extent of lung cavitation.117,119 There is a solitary case report of TB of the pancreas resulting in brittle diabetes mellitus.3
The presence of diabetes can alter the clinical presentation of TB and its outcome, in terms of delayed sputum/culture conversion, increased morbidity and mortality, and treatment failure.3,120 It is recommended to extend treatment to 9 months for patients with diabetes, cavitary disease, or delayed sputum clearance. The highest prevalence of diabetes mellitus among patients with TB is observed in Asia, North America, and Oceania; it is relatively high in Africa, and low in western countries.121 Factors associated with TB and diabetes mellitus comorbidity include sex, older age, urban residence, illicit drug use, alcoholism, cigarette smoking, sedentary lifestyle, obesity, HIV coinfection, hypertension, longstanding diabetes, poor glycemic control, pulmonary TB, and a family history of diabetes.121 Though data indicate that diabetes increases the risk of active TB, the current understanding of biologic pathways between diabetes and risk of TB is greatly limited.
Drug interaction with tuberculosis and diabetes mellitus
Rifampicin induces an acute transient hyperglycemia due to its effect of augmenting intestinal absorption of glucose.122 It also induces the cytochrome P450 enzyme, which augments the hepatic metabolism of most oral hypoglycemic agents.122 Hence, patients with co-existing diabetes and TB should have their doses of oral anti-diabetic drugs adjusted upwards according to the plasma glucose concentration. An overdose of isoniazid may cause hyperglycemia, while in rare circumstances, diabetes may become difficult to control in patients on pyrazinamide. Rarely, hypoglycemia may be seen in patients on ethionamide.
Disorders of calcium and vitamin D metabolism
Granulomatous diseases like TB are associated with hypercalcemia due to increased tissue conversion of 25-hydroxyvitamin D3 to 25-dihydroxyvitamin D3 or calcitriol.3 Hypercalcemia also may be due to the increased intestinal absorption of calcium as a result of the production of 1,25-dihydroxyvitamin D by macrophages. 1,25-dihydroxyvitamin D produced by macrophages modulates the immune response by inducing expression of antimicrobial peptides, stimulation of the autophagy pathway, and acceleration of the resolution of inflammatory responses.123 However, some studies have shown no correlation between 1,25-dihydroxyvitamin D3 levels and serum calcium measurements.123 Though most often occurring with pulmonary TB, hypercalcemia is occasionally seen in conjunction with extrapulmonary diseases like miliary TB, peritonitis, and osteomyelitis. The incidence is also higher in the presence of chronic kidney disease.
Conclusion
TB may directly or indirectly affect various endocrine glands including the adrenals, hypothalamus, pituitary, and thyroid, with the adrenal gland being most commonly affected. Extrapulmonary TB, such as that occurring in the endocrine glands, presents a diagnostic challenge due to its infrequent occurrence and presentation with nonspecific symptoms. This may result in diagnosis of the disease at an advanced stage, placing the patient at increased risk of morbidity and mortality. Thus, awareness about the occurrence of endocrine and metabolic dysfunction with TB needs to be emphasized among clinicians working in TB endemic regions. A high index of suspicion with a thorough evaluation, and if diagnosed, treatment with anti-TB therapy is necessary to improve quality of life and reduce mortality. The prevalence of, and endocrine and metabolic manifestations of, TB are summarised in Table 5.